Semi Micro Qualitative Analysis Of Simple Inorganic Salt.docx 1s2i5r
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report 3b7i
Overview 3e4r5l
& View Semi Micro Qualitative Analysis Of Simple Inorganic Salt.docx as PDF for free.
More details w3441
- Words: 957
- Pages: 14
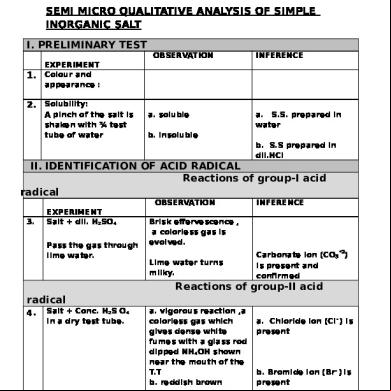
SEMI MICRO QUALITATIVE ANALYSIS OF SIMPLE INORGANIC SALT I. PRELIMINARY TEST OBSERVATION
1.
INFERENCE
EXPERIMENT Colour and appearance :
2. Solubility:
A pinch of the salt is shaken with ¾ test tube of water
a. soluble
a. S.S. prepared in water
b. insoluble b. S.S prepared in dil.HCl
II. IDENTIFICATION OF ACID RADICAL Reactions of group-I acid radical OBSERVATION 3.
EXPERIMENT Salt + dil. H2SO4 the gas through lime water.
INFERENCE
Brisk effervescence , a colorless gas is evolved. -
Lime water turns milky.
Carbonate ion (CO3 2) is present and confirmed
Reactions of group-II acid radical 4. Salt + Conc. H2S O4 in a dry test tube.
a. vigorous reaction ,a colorless gas which gives dense white fumes with a glass rod dipped NH4OH shown near the mouth of the T.T b. reddish brown vapours are evolved
a. Chloride ion (Cl- ) is present
b. Bromide ion (Br- ) is present
5.
To the above solution add copper turnings and heat
c. Violet vapours are evolved.
c. Iodide ion is present.
Reddish brown fumes are evolved and the solution turns blue.
nitrate ion (NO3-)is present
Confirmatory test for Cl-
, Br- , I-
(Silver nitrate test) 6.
S.S + dil.HNO3 + AgNO3
a. curdy white ppt soluble in excess of NH4OH b. pale yellow ppt sparingly soluble in excess of NH4OH c. yellow ppt insoluble in excess of NH4OH
-
confirmed
b. Br-
confirmed
a. Cl
c. I-
confirmed
Confirmatory test for Cl- (Chromyl chloride test) 7.
Salt+ potassium dichromate solid + Conc. H2SO4 + heat the gas that is evolved through NaOH solution. The solution is acidified with acetic acid and add lead acetate solution .
8.
Orange red vapours Solution become Yellow
Cl-
confirmed
Yellow ppt
Confirmatory test for NO3- (Brown ring test) S.S+ freshly prepared A brown ring is formed Nitrate ion (NO3-) Fe SO4 solution+ Con. H2SO4 along the sides of the test tube
at the junction of two liquids
confirmed
Reactions of group-III acid radical
OBSERVATION 9.
EXPERIMENT Test for Sulphate: S.S + BaCl2 solution.
A white ppt insoluble in dil.HCl even on heating
INFERENCE SO4
-2
present
Confirmatory test for Sulphate ion 10 .
Lead acetate test: S.S + acetic acid + lead acetate solution.
White ppt
-
SO4 2confirmed
The acid radical was found to be………………………………………………………
III. IDENTIFICATION OF BASIC RADICAL Reactions of group zero basic radical OBSERVATION
INFERENCE
EXPERIMENT 11 .
+
Test for NH4 ion: Salt + NaOH solution + boil
A pungent smelling gas which gives dense white fumes glass rod
+
NH4
ion is present
with a glass rod dipped in Conc.HCl is evolved
Confirmatory test for NH4+ ion 12 .
Nessler’s reagent test: S.S + Nessler’s reagent
Reddish brown ppt
+
NH4
ion is confirmed
Preparation of Salt solution: A concentrated solution of the given salt is prepared by dissolving it in……………………………………………………..
Group-I GroupII S.S + dil.HCl
No ppt Pb+2 absent
S.S + dil.HCl+ H2S(g)
No ppt Cu+2 absent
Group-III
Group separation chart Group-IV Group-V Group-VI
S.S + NH4Cl(s)+ NH4OH (Ex)
S.S + NH4Cl(s)+ NH4OH (Ex) + H2S(g)
S.S + NH4Cl(s)+ NH4OH (Ex) + (NH4)2CO3
No common group reagent
Gelatinous white ppt Al+3 present
Greyish white ppt Zn+2 present Buff ppt Mn+2 present Black ppt
White ppt
Mg+2 may be present
Ba+2 / Sr+2/ Ca+2 present
Ni+2 / Co+2
No ppt Al+3 absent
present No ppt +2
No ppt +2
Zn / Mn / Ni+2 / Co+2
Ba+2 / Sr+2/ Ca+2
absent
absent
Reactions of group-III basic radical OBSERVATION 13 .
EXPERIMENT S.S + NH4OH solution drop by drop to excess.
White gelatinous ppt insoluble in excess.
INFERENCE Al+3 present
Confirmatory test for Al+3 14 .
S.S + NaOH solution drop by drop to excess.
White gelatinous ppt which is soluble in excess NaOH. Al+3 confirmed
To the above solution add NH4Cl(s) and warm.
White gelatinous ppt reappears.
Reactions of group-IV basic radical 15
.
S.S + NaOH solution drop by drop.
a. white ppt soluble in excess
a. Zn+2 present b. Mn+2 present
b. white ppt insoluble in excess of NaOH turns brown on exposure to air
Confirmatory test for Zn +2 16 .
S.S+ potassium Pale green ppt ferrocyanide solution.
Zn+2 confirmed
Confirmatory test for Mn+2 17 .
S.S+ PbO2 (s)+ Con. HNO3 boil, cool and dilute with water
Supernatant solution turns pink in colour
Mn+2 confirmed
Confirmatory test for Co+2 18 .
S.S+ NH4OH solution+ acetic acid
Yellow ppt
Co+2 confirmed
solution + crystals of potassium nitrite. warm.
Confirmatory test for Ni+2 19 .
S.S+ NaOH + few drops of dimethyl glyoxime
Bright red ppt
Ni+2 confirmed
Reactions of group-V basic radical OBSERVATION 20 .
EXPERIMENT S.S+ acetic acid+ potassium chromate solution.
Yellow ppt.
INFERENCE Ba+2 present.
Confirmatory test for Ba+2 21 .
22 .
Salt + Conc.HCl .make a paste with glass rod and then introduced it into the flame. S.S + ammonium Sulphate solution.
Apple green colour is imparted to the flame.
white ppt
Ba+2 confirmed.
Sr+2 present
Confirmatory test for Sr+2 23 .
24 .
Salt + Conc.HCl .make a paste with glass rod and then introduced it into the flame S.S+ NH4OH solution+ ammonium oxalate solution.
Crimson red colour is imparted to the flame
Sr+2 confirmed
White ppt
Ca+2 present
Confirmatory test for Ca+2 25 .
Salt + Conc.HCl .make a paste with
Brick red colour is imparted to the flame
Ca+2 confirmed
glass rod and then introduced it into the flame
Reactions of group-VI basic radical 26 .
S.S + NH4Cl(s)+ NH4OH (Ex) +ammonium phosphate (scratch the inner side of the test tube with a glass rod)
Result:
White crystalline ppt
Mg+2 present and confirmed
The acid radical was found to be………………………….
The basic radical was found to be………………………… The salt is……………………………………………………
-2
SCO3
1.
INFERENCE
EXPERIMENT Colour and appearance :
2. Solubility:
A pinch of the salt is shaken with ¾ test tube of water
a. soluble
a. S.S. prepared in water
b. insoluble b. S.S prepared in dil.HCl
II. IDENTIFICATION OF ACID RADICAL Reactions of group-I acid radical OBSERVATION 3.
EXPERIMENT Salt + dil. H2SO4 the gas through lime water.
INFERENCE
Brisk effervescence , a colorless gas is evolved. -
Lime water turns milky.
Carbonate ion (CO3 2) is present and confirmed
Reactions of group-II acid radical 4. Salt + Conc. H2S O4 in a dry test tube.
a. vigorous reaction ,a colorless gas which gives dense white fumes with a glass rod dipped NH4OH shown near the mouth of the T.T b. reddish brown vapours are evolved
a. Chloride ion (Cl- ) is present
b. Bromide ion (Br- ) is present
5.
To the above solution add copper turnings and heat
c. Violet vapours are evolved.
c. Iodide ion is present.
Reddish brown fumes are evolved and the solution turns blue.
nitrate ion (NO3-)is present
Confirmatory test for Cl-
, Br- , I-
(Silver nitrate test) 6.
S.S + dil.HNO3 + AgNO3
a. curdy white ppt soluble in excess of NH4OH b. pale yellow ppt sparingly soluble in excess of NH4OH c. yellow ppt insoluble in excess of NH4OH
-
confirmed
b. Br-
confirmed
a. Cl
c. I-
confirmed
Confirmatory test for Cl- (Chromyl chloride test) 7.
Salt+ potassium dichromate solid + Conc. H2SO4 + heat the gas that is evolved through NaOH solution. The solution is acidified with acetic acid and add lead acetate solution .
8.
Orange red vapours Solution become Yellow
Cl-
confirmed
Yellow ppt
Confirmatory test for NO3- (Brown ring test) S.S+ freshly prepared A brown ring is formed Nitrate ion (NO3-) Fe SO4 solution+ Con. H2SO4 along the sides of the test tube
at the junction of two liquids
confirmed
Reactions of group-III acid radical
OBSERVATION 9.
EXPERIMENT Test for Sulphate: S.S + BaCl2 solution.
A white ppt insoluble in dil.HCl even on heating
INFERENCE SO4
-2
present
Confirmatory test for Sulphate ion 10 .
Lead acetate test: S.S + acetic acid + lead acetate solution.
White ppt
-
SO4 2confirmed
The acid radical was found to be………………………………………………………
III. IDENTIFICATION OF BASIC RADICAL Reactions of group zero basic radical OBSERVATION
INFERENCE
EXPERIMENT 11 .
+
Test for NH4 ion: Salt + NaOH solution + boil
A pungent smelling gas which gives dense white fumes glass rod
+
NH4
ion is present
with a glass rod dipped in Conc.HCl is evolved
Confirmatory test for NH4+ ion 12 .
Nessler’s reagent test: S.S + Nessler’s reagent
Reddish brown ppt
+
NH4
ion is confirmed
Preparation of Salt solution: A concentrated solution of the given salt is prepared by dissolving it in……………………………………………………..
Group-I GroupII S.S + dil.HCl
No ppt Pb+2 absent
S.S + dil.HCl+ H2S(g)
No ppt Cu+2 absent
Group-III
Group separation chart Group-IV Group-V Group-VI
S.S + NH4Cl(s)+ NH4OH (Ex)
S.S + NH4Cl(s)+ NH4OH (Ex) + H2S(g)
S.S + NH4Cl(s)+ NH4OH (Ex) + (NH4)2CO3
No common group reagent
Gelatinous white ppt Al+3 present
Greyish white ppt Zn+2 present Buff ppt Mn+2 present Black ppt
White ppt
Mg+2 may be present
Ba+2 / Sr+2/ Ca+2 present
Ni+2 / Co+2
No ppt Al+3 absent
present No ppt +2
No ppt +2
Zn / Mn / Ni+2 / Co+2
Ba+2 / Sr+2/ Ca+2
absent
absent
Reactions of group-III basic radical OBSERVATION 13 .
EXPERIMENT S.S + NH4OH solution drop by drop to excess.
White gelatinous ppt insoluble in excess.
INFERENCE Al+3 present
Confirmatory test for Al+3 14 .
S.S + NaOH solution drop by drop to excess.
White gelatinous ppt which is soluble in excess NaOH. Al+3 confirmed
To the above solution add NH4Cl(s) and warm.
White gelatinous ppt reappears.
Reactions of group-IV basic radical 15
.
S.S + NaOH solution drop by drop.
a. white ppt soluble in excess
a. Zn+2 present b. Mn+2 present
b. white ppt insoluble in excess of NaOH turns brown on exposure to air
Confirmatory test for Zn +2 16 .
S.S+ potassium Pale green ppt ferrocyanide solution.
Zn+2 confirmed
Confirmatory test for Mn+2 17 .
S.S+ PbO2 (s)+ Con. HNO3 boil, cool and dilute with water
Supernatant solution turns pink in colour
Mn+2 confirmed
Confirmatory test for Co+2 18 .
S.S+ NH4OH solution+ acetic acid
Yellow ppt
Co+2 confirmed
solution + crystals of potassium nitrite. warm.
Confirmatory test for Ni+2 19 .
S.S+ NaOH + few drops of dimethyl glyoxime
Bright red ppt
Ni+2 confirmed
Reactions of group-V basic radical OBSERVATION 20 .
EXPERIMENT S.S+ acetic acid+ potassium chromate solution.
Yellow ppt.
INFERENCE Ba+2 present.
Confirmatory test for Ba+2 21 .
22 .
Salt + Conc.HCl .make a paste with glass rod and then introduced it into the flame. S.S + ammonium Sulphate solution.
Apple green colour is imparted to the flame.
white ppt
Ba+2 confirmed.
Sr+2 present
Confirmatory test for Sr+2 23 .
24 .
Salt + Conc.HCl .make a paste with glass rod and then introduced it into the flame S.S+ NH4OH solution+ ammonium oxalate solution.
Crimson red colour is imparted to the flame
Sr+2 confirmed
White ppt
Ca+2 present
Confirmatory test for Ca+2 25 .
Salt + Conc.HCl .make a paste with
Brick red colour is imparted to the flame
Ca+2 confirmed
glass rod and then introduced it into the flame
Reactions of group-VI basic radical 26 .
S.S + NH4Cl(s)+ NH4OH (Ex) +ammonium phosphate (scratch the inner side of the test tube with a glass rod)
Result:
White crystalline ppt
Mg+2 present and confirmed
The acid radical was found to be………………………….
The basic radical was found to be………………………… The salt is……………………………………………………
-2
SCO3